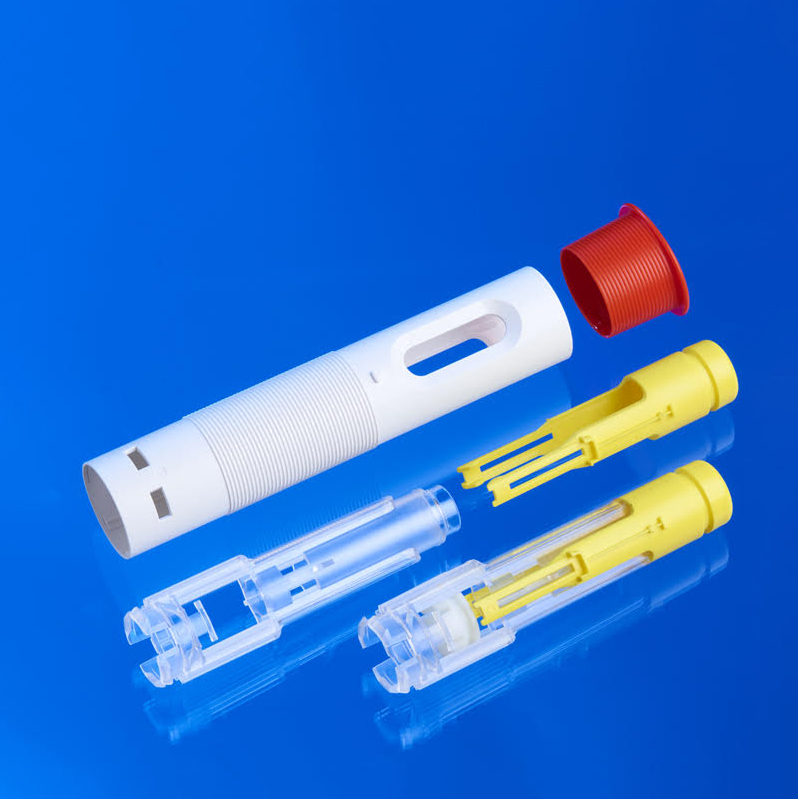
As medical devices continue to evolve in complexity, functionality, and user-centric design, the demand for integrated multi-material solutions has surged. One process at the forefront of this evolution is 2K injection molding—a highly controlled, precision manufacturing method enabling the production of dual-material or dual-color components in a single tool cycle. For regulated markets such as drug delivery, diagnostics, ophthalmics, and minimally invasive technologies, 2K molding delivers critical advantages in form, function, and regulatory compliance.
What is 2K Molding?
Also referred to as two-shot injection molding, 2K molding is a fully automated process where two distinct polymers are injected sequentially or simultaneously into a single mold. These materials may differ in rigidity, color, chemical resistance, or tactile properties—allowing for high-performance, single-assembly components without secondary bonding or assembly steps.
This process is distinct from traditional overmolding, as 2K molding occurs within the same machine cycle, using rotating platen, core-back, or index plate systems to position the substrate and allow precise overmolding of the secondary material.
Medical Applications of 2K Molding
2K molding is particularly suited to medical and pharmaceutical device manufacturing, where precision, cleanability, chemical resistance, and user interaction are all critical. Common applications include:
- Auto-injectors with rigid internal structure and soft overmolded grips
- Inhaler bodies combining transparent viewing windows with robust housing
- IV connectors and valves with integrated seals
- Wearable drug delivery systems with dual-material enclosures and interfaces
- Ophthalmic packaging with compliant closures and rigid optical zones
- Microfluidic diagnostic cartridges integrating optical windows and fluidic pathways
Each of these applications benefits from permanent bonding, biocompatibility, and sterility assurance provided by 2K molding under validated cleanroom conditions.
Key Technical Advantages
1. Precision Material Bonding
With tool-controlled polymer interface formation, the process ensures strong interlayer adhesion without adhesives. This is crucial for parts requiring leak-tight seals, consistent mechanical interfaces, or optical clarity across polymer boundaries.
2. Regulatory-Compliant Cleanroom Integration
For medical applications, 2K processes can be fully validated (FAT, IQ, OQ, PQ) and executed in ISO Class 7 and 8 cleanroom environments, as required under ISO 13485 and FDA QSR guidelines.
3. Design for Function
2K allows engineers to design for tactile function (e.g., grip, actuation), patient safety (e.g., color coding), or integrated mechanical properties—without sacrificing dimensional accuracy or increasing part count.
4. Reduced Bioburden and Contamination Risk
By eliminating assembly and adhesive bonding, 2K molding reduces contamination risk and minimizes the need for downstream aseptic processing, a major benefit in diagnostics and implantables.
5. Cost and Supply Chain Efficiency
Combining parts during molding reduces labor, assembly, and logistics—delivering lower total part cost and shorter time to market for validated, patient-ready assemblies.
Materials Selection in 2K Molding
Choosing compatible materials is critical. Common medical-grade pairings include:
- PC + TPE (rigid/soft) for ergonomic enclosures
- COC + PP for diagnostic cartridges
- PC + ABS for structural and aesthetic integration
- PEEK + TPU for high-temperature or implantable components
Each pairing must be vetted for chemical bonding compatibility, sterilization resistance, and biocompatibility (USP Class VI or ISO 10993) depending on device classification.

Tooling and Process Requirements
Effective 2K molding demands advanced tooling and tight process control:
- Multi-component mold design (rotary platen, core-back, or transfer systems)
- Dual-barrel injection molding machines with precise temperature and pressure regulation
- Advanced simulation tools (e.g., Moldflow) to analyze interface bonding and shrinkage
- Tooling tolerances in the micron range to maintain part integrity and regulatory compliance
- In-mold robotics for part transfer or insert placement
2K Molding at Optimold
Optimold, together with its sister company Micro System offer a fully integrated, ISO 13485-certified manufacturing platform for medical-grade 2K molding, with world-class capabilities in:
- Ultra-precision mold tooling (sub-micron accuracy)
- 2K injection molding from 30t to 300t, with all-electric and twin-shot machines
- ISO Class 7 cleanroom production for regulated medical products
- Comprehensive validation protocols: FAT, IQ, OQ, PQ
- Advanced metrology and optical inspection for complex micro features
- 24/5 and 24/7 production availability across UK and Singapore facilities
By integrating design, tooling, validation, and production under one group, we reduce risk, speed up development, and support long-term manufacturing scalability for global OEMs.
2K injection molding has become a critical enabler of next-generation medical and diagnostic devices. It supports the integration of multiple functions, improves patient usability, and reduces assembly risks—all within a fully validated, cleanroom-compliant process.
When executed by a qualified, ISO 13485-certified partner, 2K molding delivers measurable value across product performance, regulatory assurance, and commercial viability—making it a smart investment for forward-thinking medical OEMs.
Contact us today!