ISO 13485 Injection Molding for Medical Devices
Optimold delivers advanced injection molding solutions tailored to the medical device industry. With over 15 years of experience and ISO 13485 certification, we understand the strict standards of medical manufacturing. Our cleanroom injection molding ensures top-tier cleanliness and quality, while our on-site tooling partner, Micro Systems, enables faster turnarounds and greater design flexibility. Combining precision, efficiency, and compliance, we help medical device manufacturers bring complex, high-performance products to market with confidence.
We manufacture your medical devices
For over a decade, Optimold has delivered high-quality injection molding solutions for a wide range of medical device applications, including Class I, II, and III components. We work with various medical-grade resins and utilize ISO 7 and 8 cleanroom environments to ensure top-tier cleanliness and compliance. Our advanced equipment, automated inspection systems, and scientific molding processes support full quality assurance—from mold design and validation to production. With IQ, OQ, PQ validations and lot traceability, Optimold meets the strict quality standards required for medical device manufacturing.
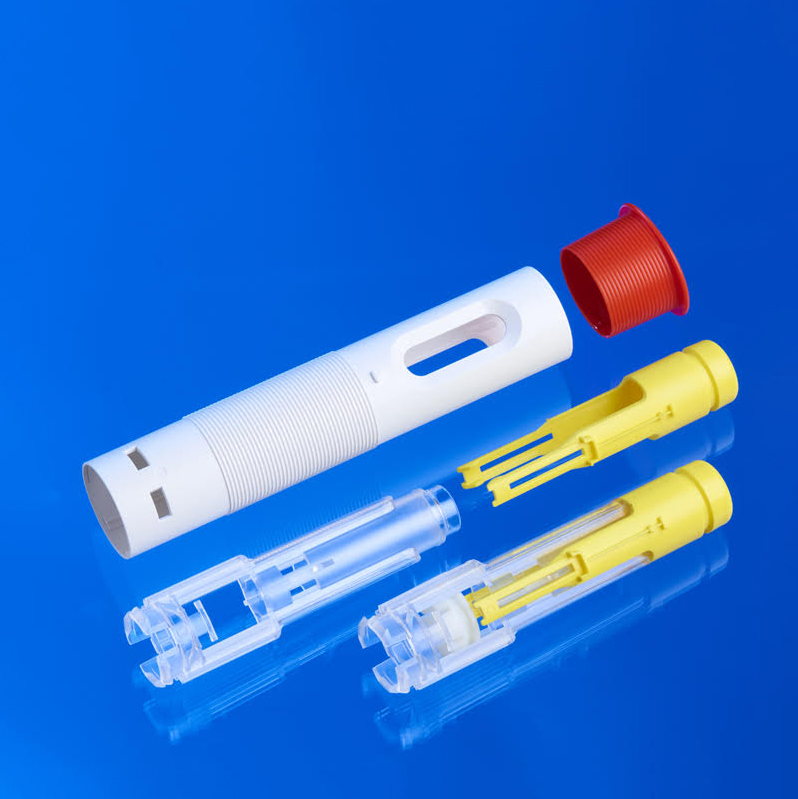
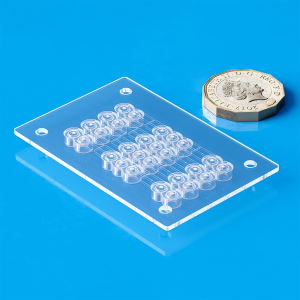
Medical Device Injection Molding examples
PEEK bone implants of ……..mm, offering high strength, biocompatibility, and radiolucency—ideal for orthopedic use.
Capillary clamps of ……mm, precision-molded to control and secure microfluidic channels in diagnostic and medical devices, ensuring accurate fluid flow.
Micro tube connectors of …..m, injection molded to securely join small-diameter tubing in medical and diagnostic devices, ensuring reliable fluid transfer.
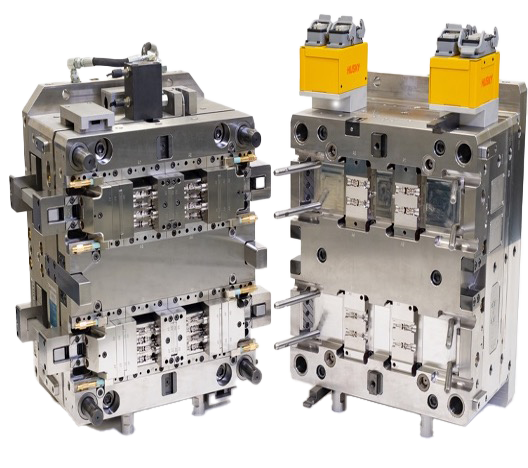
In-house Mold Tooling
In-house mold tooling is critical to achieving the precision, speed, and compliance required in medical device manufacturing. Through our on-site partner, Micro Systems—certified to ISO 9001 and ISO 13485—we offer comprehensive tool design, creation, and maintenance services. This integrated approach enables tight control over tolerances, rapid design modifications, and full traceability throughout the tooling lifecycle. By keeping tooling operations on-site, we ensure seamless alignment with part specifications, reduced lead times, and consistent quality for high-performance medical components.
Beyond Medical Device Injection Molding
Optimold provides industry-leading customer service and support for your Medical Device projects, including:
- Precision Injection Molding: high-precision molding, 2K-molding, …
- A range of high-performance engineered materials: PEEK, ABS,…
- Scalable solutions: from small lot to multi-million unit batches
- In-house tooling: ultra-precision mold (single and multi-cavity, micro mold)
- Quality Management: Validation services: FAT to full IQ, OQ, PQ – Mold testing and evaluation
- Extensive Metrology capabilities
Synergy to streamline the entire Development and Validation process
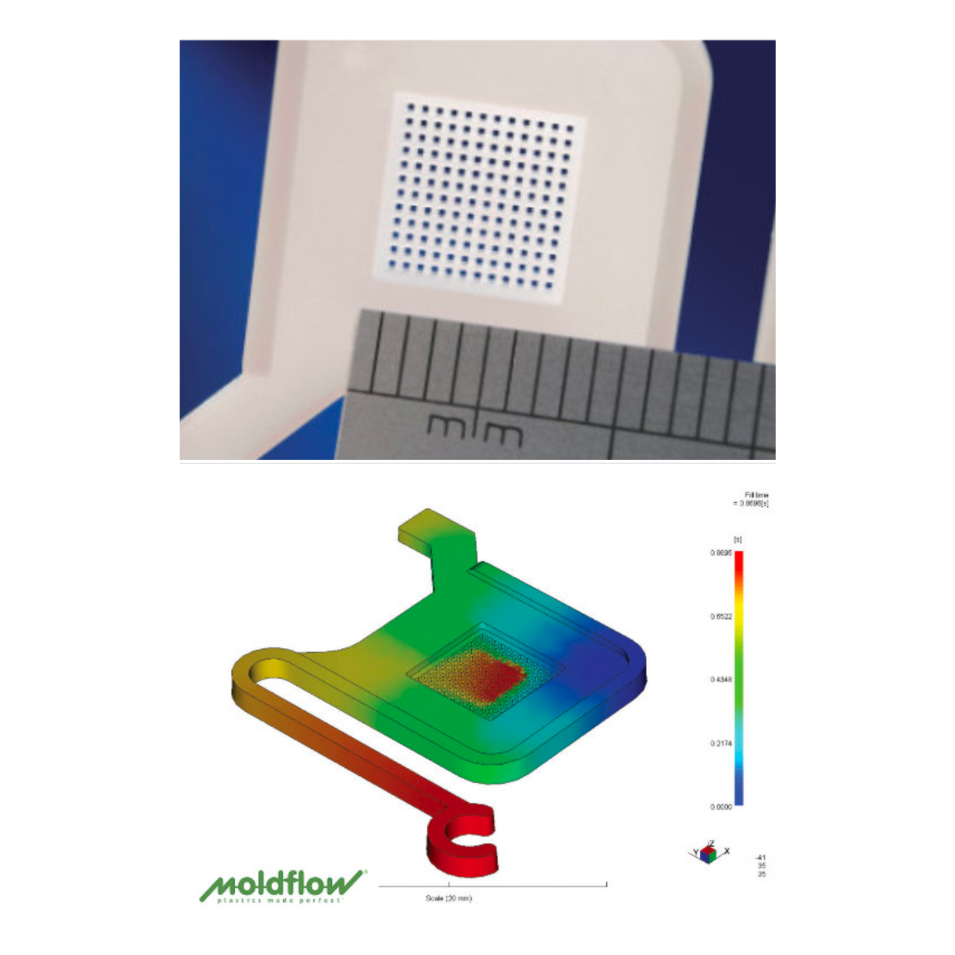
Between Optimold and Micro Systems, we provide complete manufacturing solutions unrivalled by any other mold tooling and injection molding company in the industry. Projects typically begin with a development phase starting at Design for Manufacturability (DfM), driven by DFMEA, followed by pilot or pre-production runs using a 2-cavity mold. This stage validates the design, proves functionality, and identifies issues early—minimizing risk and cost. Once successful, the process scales to full production tooling—commonly 8, 16, or 32 cavities—enabling efficient, high-volume manufacturing while maintaining quality and compliance.

Medical Mold Design
Significant expertise in Mold Design for complex Medical Device systems to ensure efficient and effective production, driven by a DFMEA approach to ensure that only the most optimized solutions are selected
Medical Mold Manufacturing
World-class tool room with the very highest accuracy manufacturing equipment to achieve sub-micron accuracy for the most reliable and efficient injection molds
Mold Testing & Development
Comprehensive mold development utilizing Pro-Op, Minitab and Cap Studies to provide the most robust molding processes and highest achievable capability
Validation
Complete validation of mold tools and molding process through IQ, OQ and PQ phases complying to ISO 13485 accreditation
Metrology
Extensive in-house metrology lab including CT Scanning with GOM licenses to efficiently generate complete metrology reports
Production Molding
ISO 13485 accredited production molding facility with 20 IMMs from 15T to 300T including multiple 160T 2-shot IMMs
Explore other applications
Talk to us about your Medical Device project
Ready to accelerate your medical device production with precision and efficiency? Partner with Optimold for ISO 13485-certified, cleanroom injection molding and integrated tooling solutions.