Ensuring Quality Through Mold Testing & Evaluation
Comprehensive Mold Testing and Evaluation facility with dedicated engineers utilising Pro-Op, Minitab and Cap Studies to provide the most robust moulding processes and highest achievable capability
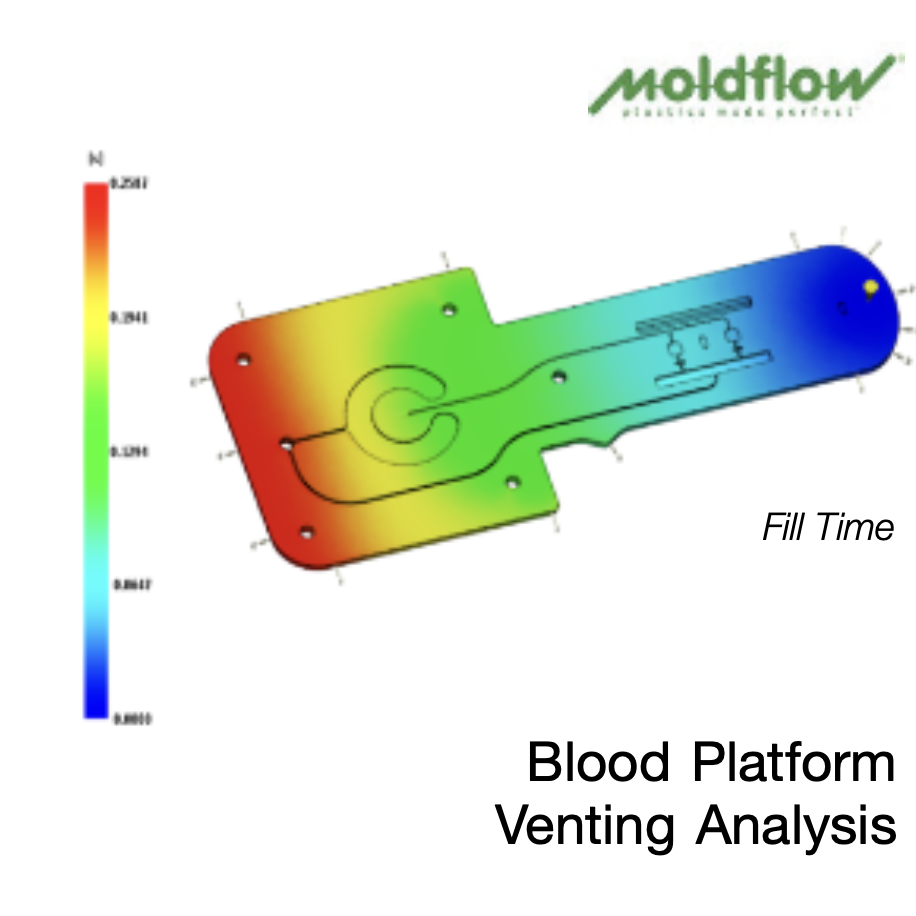
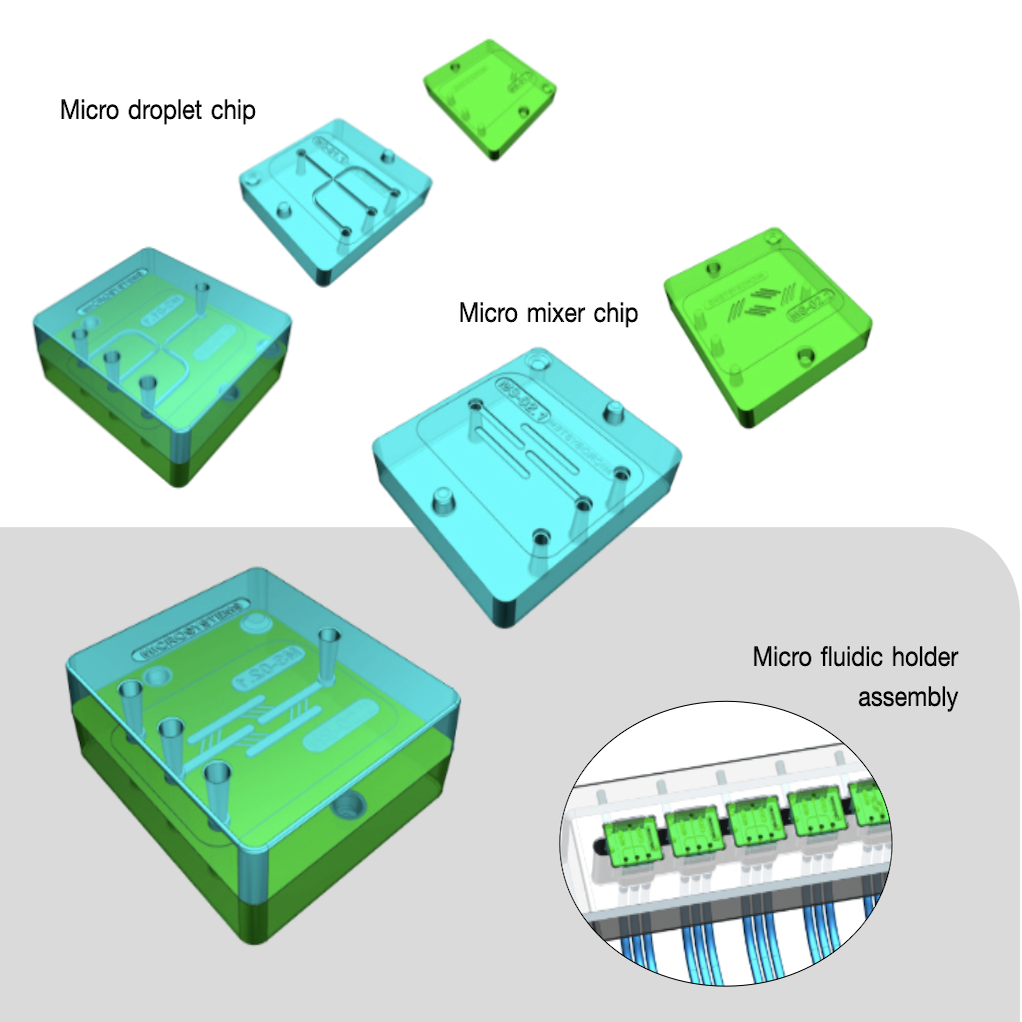
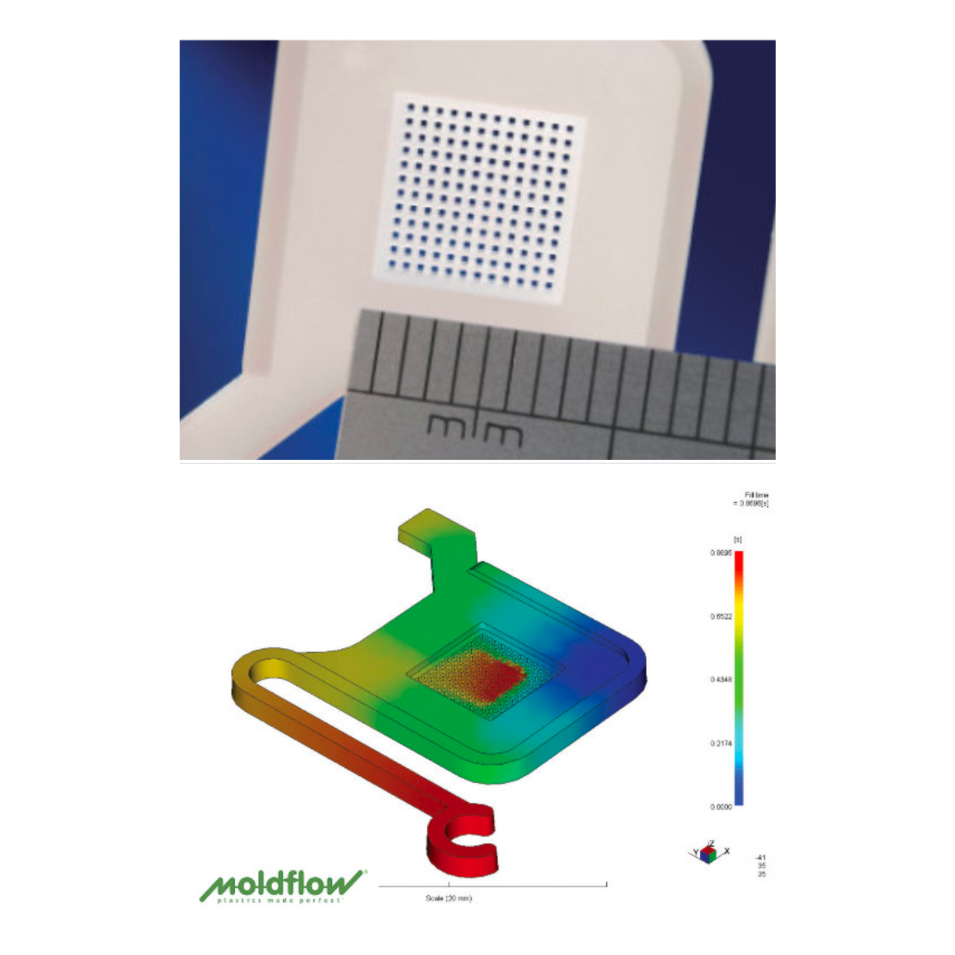
The Vital Role of Mold Testing & Evaluation
Mold testing and evaluation are essential for precision-driven industries like medical injection molding. Performed after mold fabrication and before full-scale production, mold trials validate design integrity and optimize processing parameters. In medical applications—where tight tolerances, material consistency, and regulatory compliance are non-negotiable—this step ensures molds meet exacting standards. Even slight deviations can compromise product performance and patient safety. Thorough evaluation mitigates risk, ensures repeatability, and supports efficient, compliant, and high-yield production environments.
- Early mold issue detection
- Process optimization
- Tight medical tolerances
- Fewer defects and waste
- Stable, repeatable runs
- Higher efficiency and yield
The ultimate Mold Testing & Evaluation service
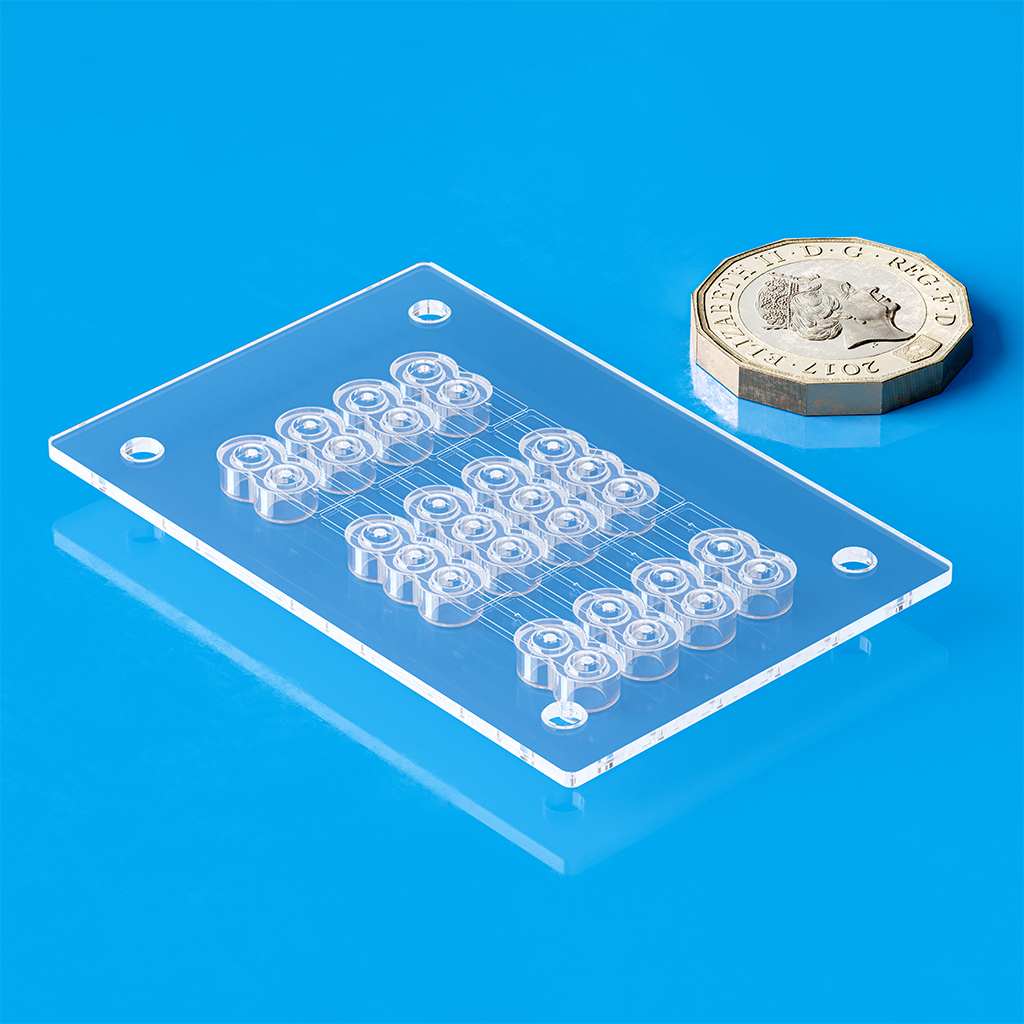
In partnership with Micro Systems, Optimold develops closed-loop multi-cavity molds that monitor and adjust melt temperatures in each cavity, ensuring high part quality and process consistency. Our tooling ranges from single-cavity pilot molds to multi-cavity systems for high-volume production.
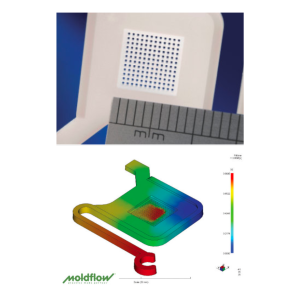
Mold testing is performed in a dedicated facility, separate from tool manufacturing, enabling confidential FAT, DOE, and steel-safe adjustments. Our engineers apply Pro-Op, Minitab, and Cap Studies to optimize process capability.
A cleanroom product testing area supports customer-specific validation, including turnkey setups with client-supplied machines—delivering robust, compliant, and production-ready solutions.
Mold Testing & Evaluation for various applications
Optimold specializes in comprehensive mold testing and evaluation services that comply with ISO 13485 standards. Rigorous testing ensures molds achieve precise, consistent performance—critical for medical, pharmaceutical, and other high-precision industries, leading to improved product quality, regulatory compliance, and cost-effective manufacturing across various sectors.
Synergy to streamline the entire Development and Validation process
At Optimold and Micro Systems, we deliver complete end-to-end manufacturing solutions that go far beyond standard injection molding services. True success—especially at scale—relies on getting the entire process right from the start. Our projects begin with Design for Manufacturability (DfM) guided by DFMEA, followed by pilot runs using 2-cavity molds to validate design, prove functionality, and catch issues early—reducing risk and cost. Only then do we scale to full production tooling, typically 8, 16, or 32 cavities, enabling efficient, high-volume manufacturing with consistent quality and compliance. This integrated approach ensures your project is built for long-term success.
- DFMEA
- Mold Manufacture
- Mold Testing & Development
- Validation
- Metrology
- Production Molding
Explore other services
Talk to our expert about your Mold Testing & Evaluation requirements
Get expert ISO 13485 Mold Testing and Evaluation to boost your injection molding project’s quality and efficiency