ISO 13485 Ultra-Precision Injection Molding Component Development
Turnkey medical component development—from DFM and precision design to mold manufacture, FAT, ISO 13485-compliant validation, and secure component supply. Our process ensures reliable, regulatory-ready production from concept to market.



Why choose us?
Complete in-house Component Development process
Extensive Metrology facilities
Extensive DFM and process optimization experience within Micro Systems.
Time-saving and cost-saving
Optimized Component Development for Injection Molding
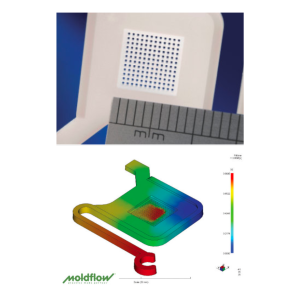
At Optimold, our component development process is designed to identify and resolve unforeseen design issues early, ensuring parts are fit for purpose and optimized for injection molding. We leverage in-house tooling and a wide range of thermoplastic materials and colors to support rapid iteration. With everything under one roof—from molding and CT scanning to immediate tool modifications with Micro Systems—we drastically reduce turnaround times. This integrated approach allows us to refine both component and mold design quickly, accelerating development and minimizing delays.
Component Development necessary for various applications
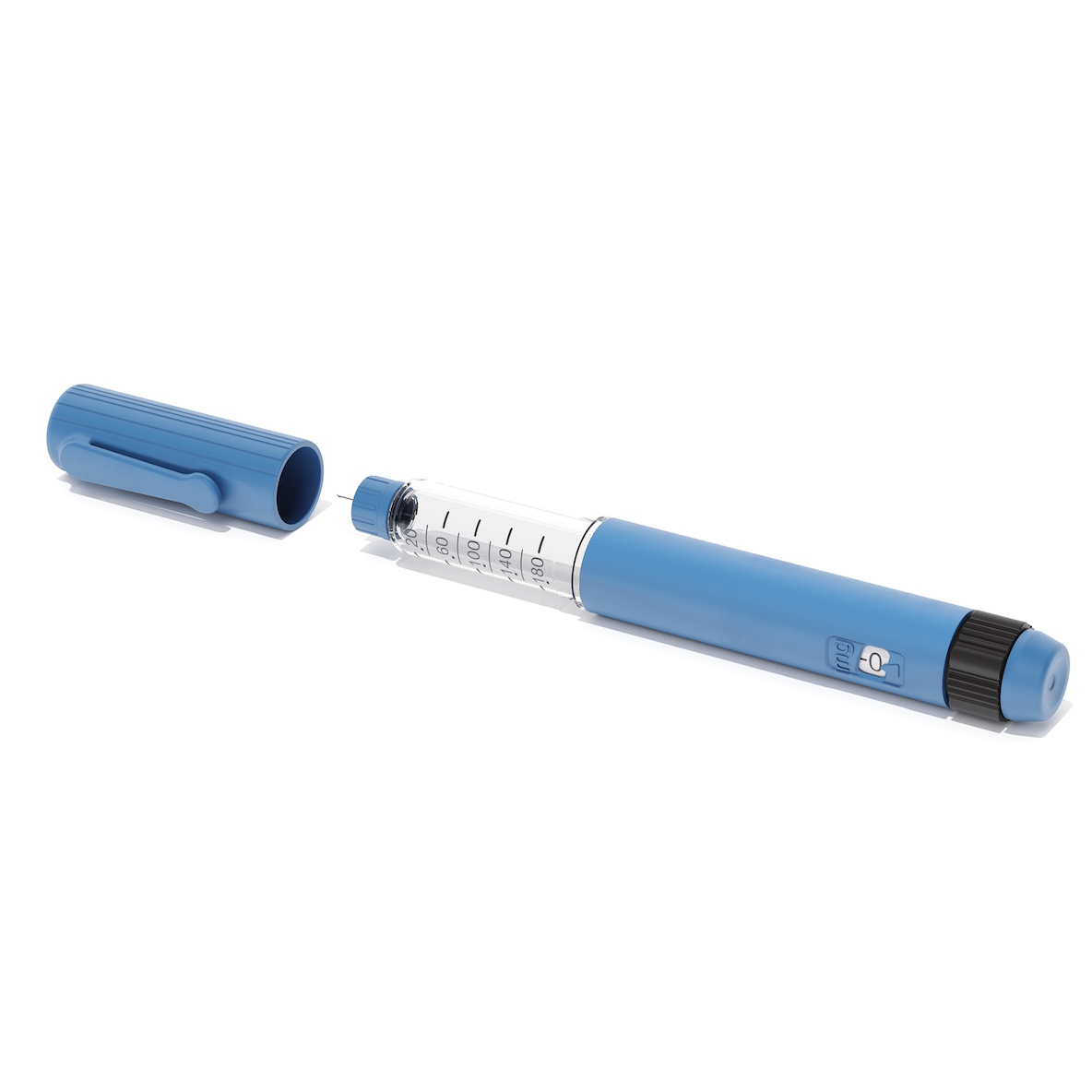
Component development is essential across industries requiring the highest precision and quality standards. With over 10 years of experience and an ISO 13485-certified quality management system, we ensure components meet stringent regulatory and performance requirements. The tool trial process further enhances development by allowing exploration of different material options, helping you evaluate finish and performance early—ensuring the final product is optimized for its intended application.
Synergy to streamline the entire Development and Validation process
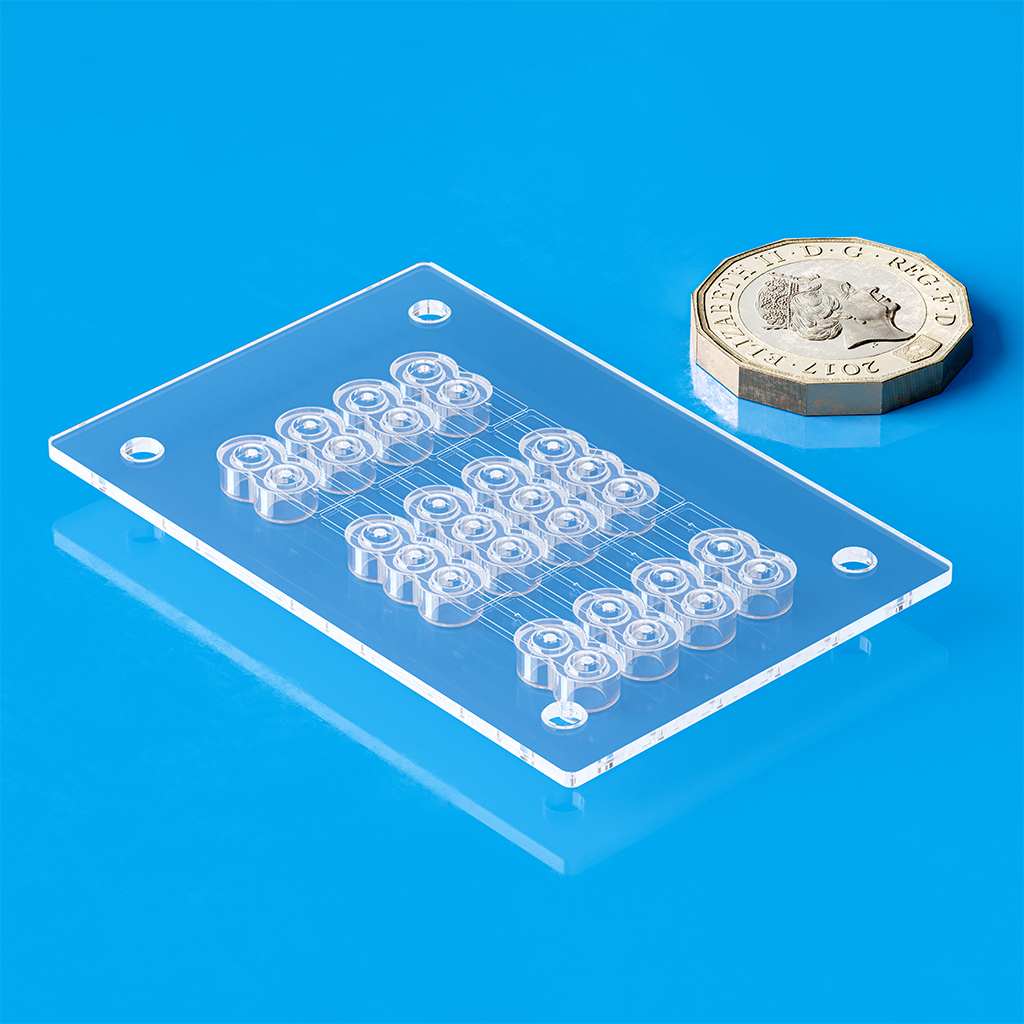
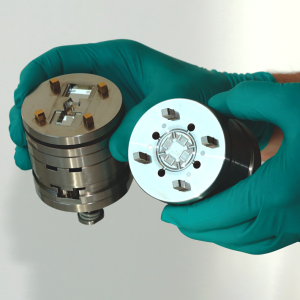
At Optimold and Micro Systems, we deliver complete end-to-end manufacturing solutions that go far beyond standard injection molding services. True success—especially at scale—relies on getting the entire process right from the start. Our projects begin with Design for Manufacturability (DfM) guided by DFMEA, followed by pilot runs using 2-cavity molds to validate design, prove functionality, and catch issues early—reducing risk and cost. Only then do we scale to full production tooling, typically 8, 16, or 32 cavities, enabling efficient, high-volume manufacturing with consistent quality and compliance. This integrated approach ensures your project is built for long-term success.
- DFMEA
- Mold Manufacture
- Mold Testing & Development
- Validation
- Metrology
- Production Molding
Explore other services
Talk to our experts to bring your components from concept to reality
Partner with us for end-to-end component development backed by ISO 13485-certified processes. From concept to validated production, we ensure precision, compliance, and performance every step of the way