Your trusted ISO 13485 Injection Molding partner for Drug Delivery Devices
Optimold delivers advanced injection molding solutions for drug delivery devices, backed by 15+ years of experience and ISO 13485 certification. We ensure tight tolerances and top-quality components—critical for patient safety and successful outcomes. Our cleanroom facilities and partnership with Micro Systems enable fast turnarounds and flexible tooling, helping manufacturers bring reliable, high-performance devices to market efficiently.
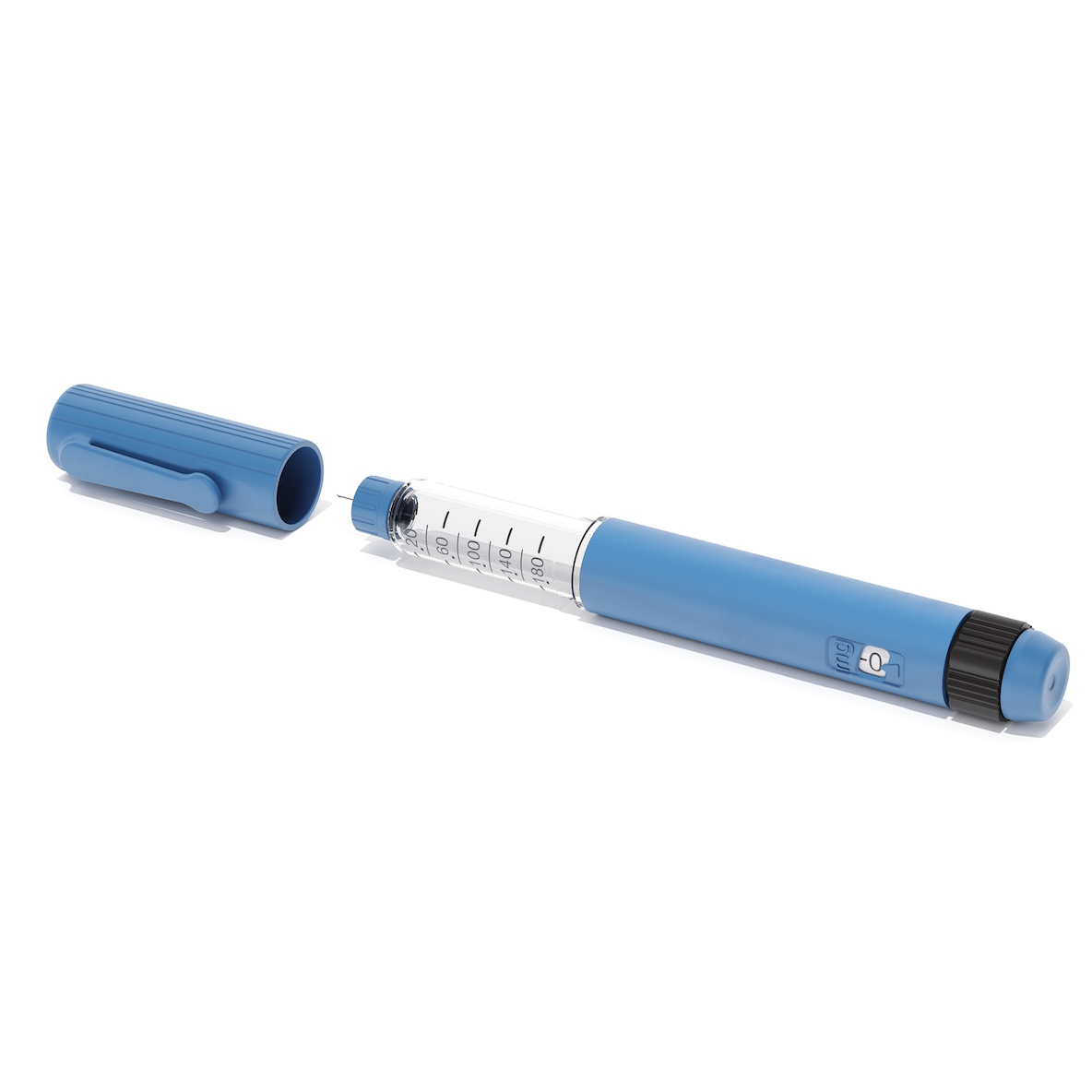
Precision Molding for Drug Delivery
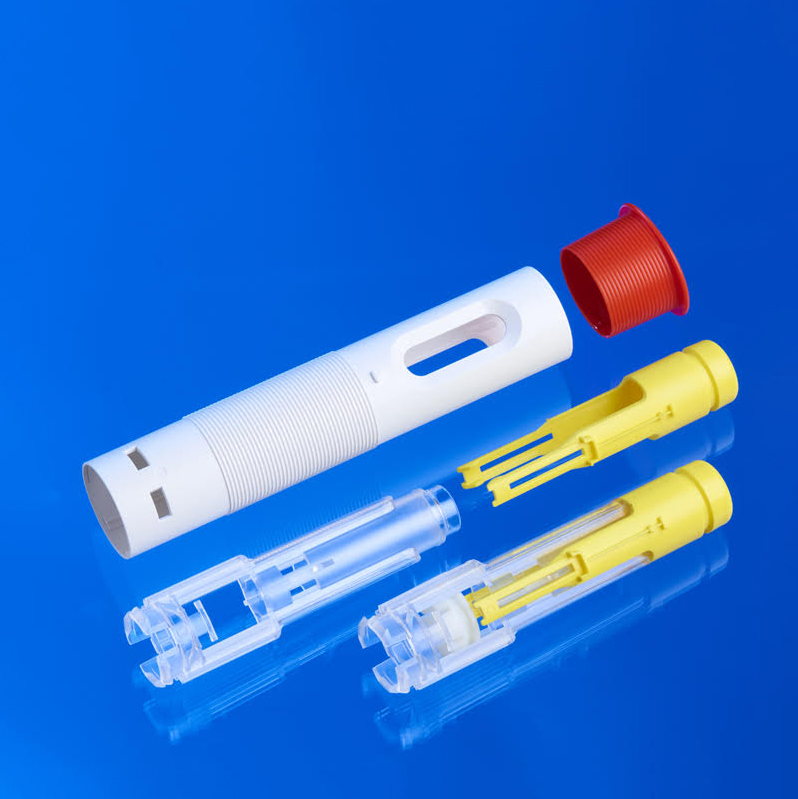
Medical Device Injection Molding examples
Quality excellence for Turnkey manufacturing solutions
We offer end-to-end expertise—from mold design to precision injection molded components—leveraging the combined capabilities of Optimold and Micro Systems. Supporting the full product lifecycle, we guide customers from early R&D and prototyping to validation, production, and market launch. Our customer-focused approach is backed by robust quality systems, Class 7 and 8 cleanroom facilities, and ISO-aligned standards. With in-house tooling from Micro Systems, we deliver reliable, high-performance tools that ensure absolute safety and consistency. Through ongoing improvement, collaboration, and deep technical expertise, we meet and exceed the highest regulatory and quality expectations.
Beyond Drug Delivery Device Injection Molding
- Precision Injection Molding: high-precision molding, 2K-molding, …
- A range of high-performance engineered materials: PEEK, ABS,…
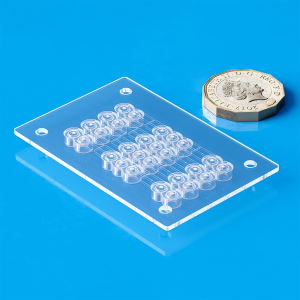
- Scalable solutions: from small lot to multi-million unit batches
- In-house tooling: ultra-precision mold (single and multi-cavity, micro mold)
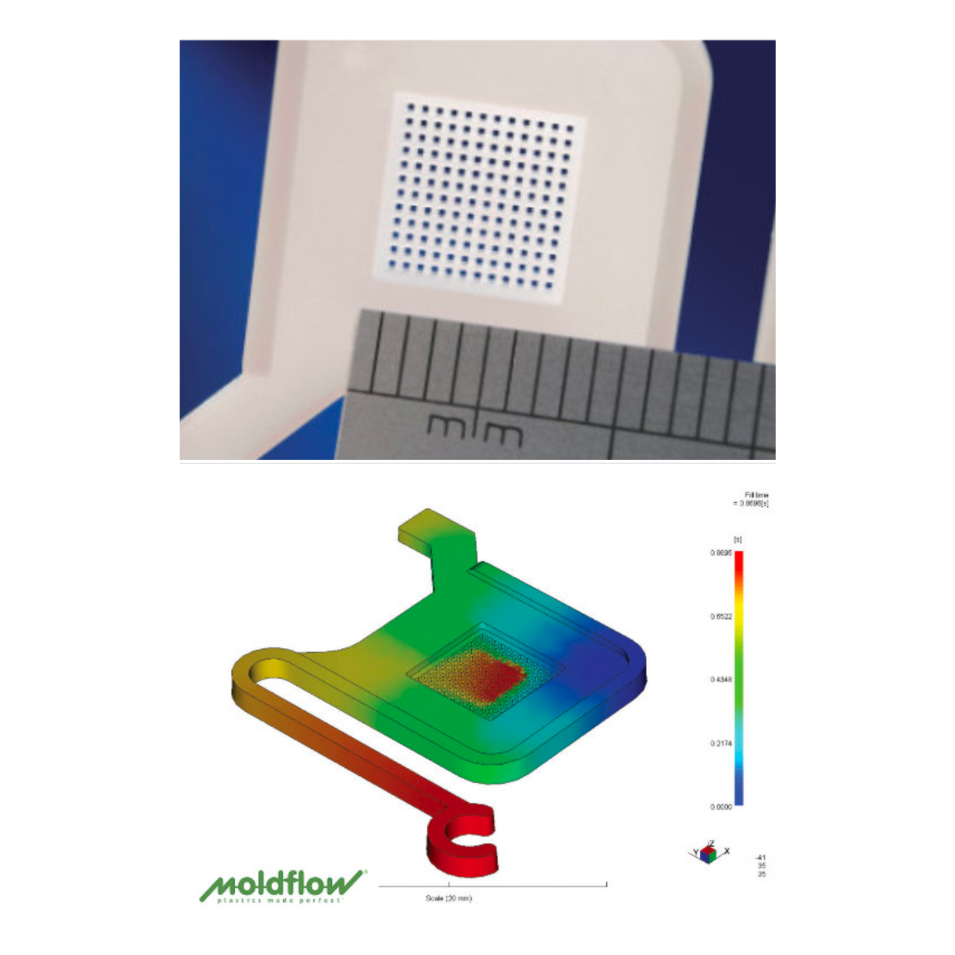
- Quality Management: Validation services: FAT to full IQ, OQ, PQ – Mold testing and evaluation
- Extensive Metrology capabilities
Synergy to streamline the entire Development and Validation process