Comprehensive ISO 13485-accredited Mold & Process Validation
Optimold provides complete validation of mold tools and molding processes through IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) phases, ensuring full compliance with ISO 13485 standards



ISO 13485 IQ-OQ-PQ Validation
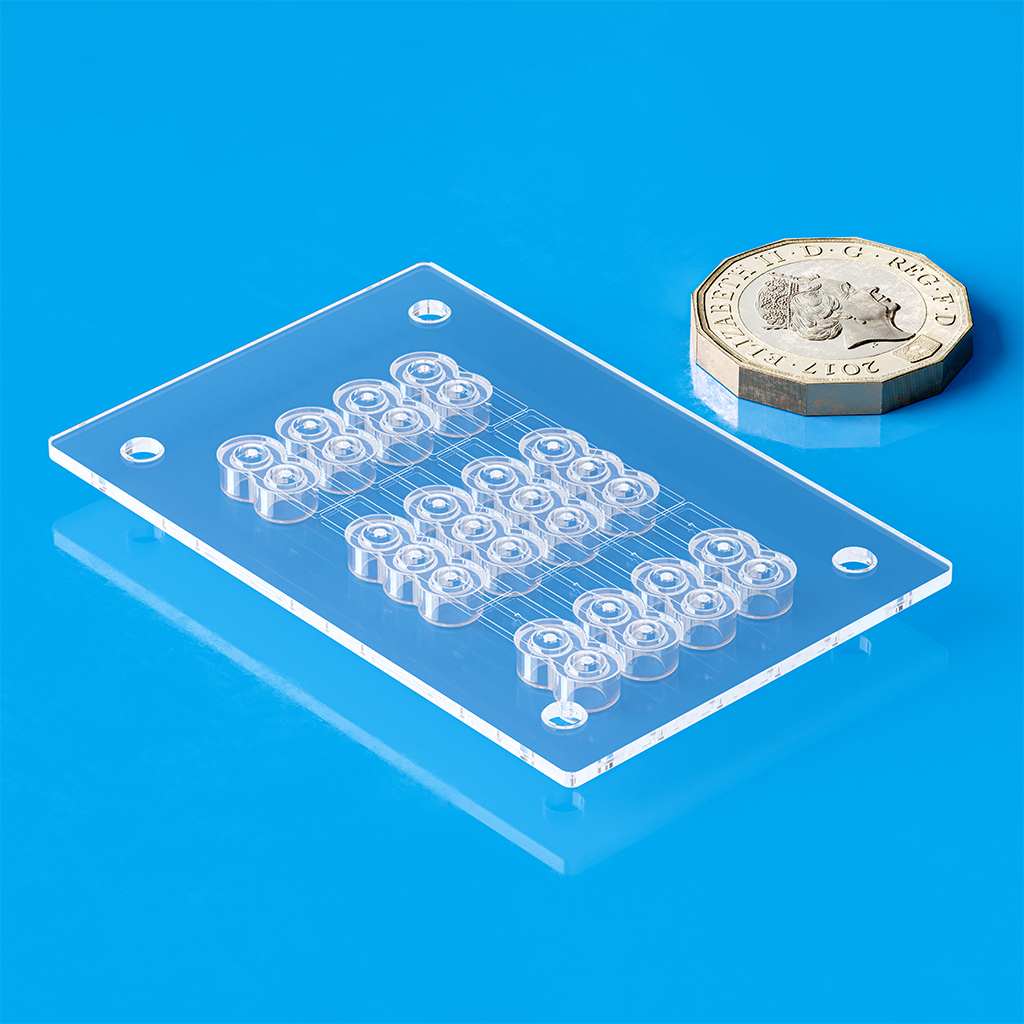
In medical device manufacturing, process validation is essential to ensure repeatability, dimensional accuracy, and consistent part quality. A fully validated injection molding process—executed through IQ, OQ, and PQ phases in compliance with ISO 13485—results in a stable, tightly controlled system with minimal variation and reduced rejects from build to build. This level of precision is critical to meeting the stringent requirements of the medical industry.
-
IQ: Confirms proper installation of molds and equipment.
-
OQ: Verifies operation within set parameters.
-
PQ: Validates consistent production of quality parts under normal conditions.
Metrology-driven IQ, OQ, PQ Validation for Critical Molding Processes
Optimold ensures high-quality injection-molded components using advanced metrology and operates under a certified ISO 13485 quality management system. We provide end-to-end mold validation services, including Factory and System Acceptance Testing and full IQ, OQ, and PQ process validation. In collaboration with Micro Systems, we develop tailored validation protocols to meet specific regulatory requirements. With hundreds of completed validations, we offer proven expertise to streamline compliance and accelerate project timelines.
Full process validation for various applications
The IQ, OQ, and PQ validation phases are critical across industries—especially in medical manufacturing—ensuring molds and processes meet stringent requirements. In injection molding, process validation aims to identify a mold-process combination capable of reliably achieving precise part dimensions and tolerances with repeatability throughout production.
Synergy to streamline the entire Development and Validation process
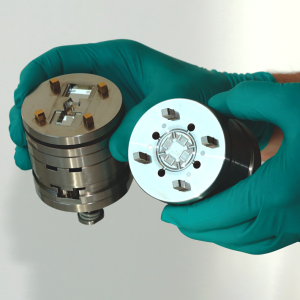
At Optimold and Micro Systems, we deliver complete end-to-end manufacturing solutions that go far beyond standard injection molding services. True success—especially at scale—relies on getting the entire process right from the start. Our projects begin with Design for Manufacturability (DfM) guided by DFMEA, followed by pilot runs using 2-cavity molds to validate design, prove functionality, and catch issues early—reducing risk and cost. Only then do we scale to full production tooling, typically 8, 16, or 32 cavities, enabling efficient, high-volume manufacturing with consistent quality and compliance. This integrated approach ensures your project is built for long-term success.
- DFMEA
- Mold Manufacture
- Mold Testing & Development
- Validation
- Metrology
- Production Molding
Explore other services
Talk to our experts about your ISO 13485 Validation requirements
Receive your complete validation of mold tools and molding process through IQ, OQ and PQ phases complying to ISO13485 accreditation today