The Importance of Validation in Injection Molding for Medical Applications
Injection molding plays a critical role in manufacturing high-precision plastic components used across medical devices, diagnostics, drug delivery systems, and ophthalmics. However, due to the highly regulated nature of the medical industry, ensuring product quality and compliance extends far beyond the molding process itself. This is where validation becomes indispensable.
What is Validation in Injection Molding?
Validation is a documented process that confirms an injection molding operation consistently produces components meeting predetermined quality and performance specifications. It involves verifying the entire production workflow—from design, tooling, and materials, to the molding machine settings and final inspection—aligns with regulatory standards and customer requirements.

Why is Validation Critical for Medical Injection Molding?
Medical components must adhere to stringent safety, reliability, and biocompatibility standards, often governed by ISO 13485 and FDA regulations. Validation ensures:
- Consistent Product Quality: Validating process parameters guarantees each batch meets dimensional, mechanical, and functional specifications.
- Regulatory Compliance: Thorough documentation and testing help satisfy audit requirements by regulatory bodies.
- Risk Mitigation: Early identification and control of potential defects prevent costly recalls and patient safety issues.
- Process Optimization: Validation activities reveal process variability, enabling continuous improvement and cost efficiency.
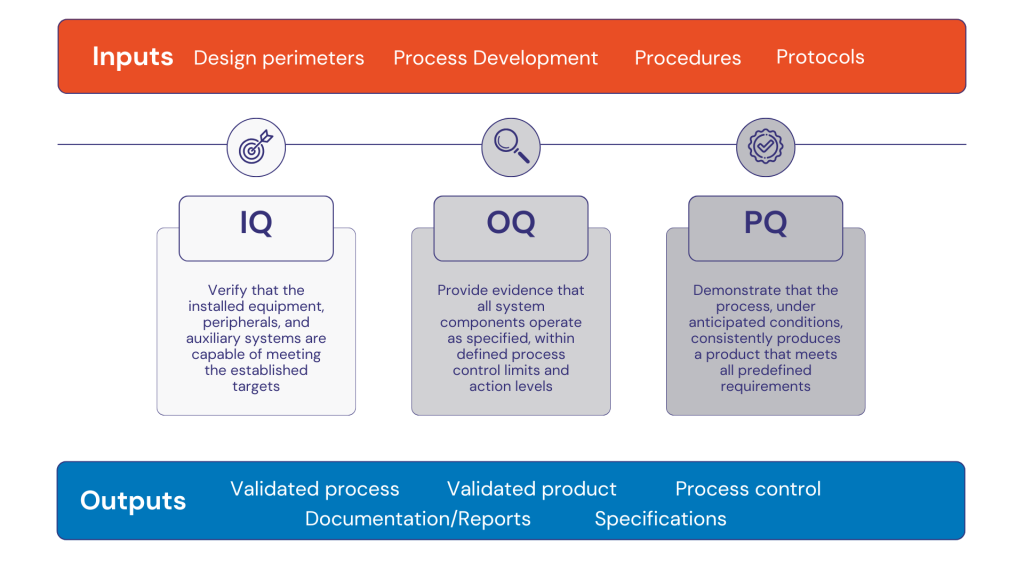
Key Validation Steps in Medical Injection Molding
- Design Validation: Confirming mold designs and materials meet medical-grade specifications.
- Installation Qualification (IQ): Verifying machines and tooling are correctly installed.
- Operational Qualification (OQ): Testing machines operate within set parameters under simulated production conditions.
- Performance Qualification (PQ): Running full production batches to demonstrate consistent quality and repeatability.
- Ongoing Process Monitoring: Using metrology and statistical process control (SPC) to maintain validated conditions.
The Role of Metrology and Cleanroom Environments
Advanced metrology tools enable precision measurement of critical dimensions and surface finishes, crucial for parts like microfluidic chips or ophthalmic components. Moreover, validated cleanroom molding environments (ISO Class 7 or 8) reduce contamination risks, essential for sterile medical devices.
Validation at Optimold
At Optimold, together with our sister company Micro Systems, validation is integral to our medical injection molding operations. Our ISO 13485-certified facility is equipped with state-of-the-art metrology instruments and cleanroom environments to ensure precision and contamination control. We follow rigorous Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols tailored to each client’s specifications. This comprehensive approach guarantees consistent product quality, regulatory compliance, and minimized risk throughout the manufacturing lifecycle. By integrating continuous monitoring and detailed documentation, Optimold delivers reliable, validated medical components that meet the highest industry standards.

Validation is not just a regulatory checkbox; it is a strategic necessity in medical injection molding. It provides assurance that complex medical components are manufactured safely, reliably, and efficiently. By investing in robust validation protocols, medical device manufacturers can accelerate time-to-market while upholding the highest standards of patient safety and product excellence.
Contact us today!







