Medical Overmolding: Precision Meets Performance in Device Manufacturing
In modern medical device design, functionality, ergonomics, and patient safety are more interconnected than ever. One technology enabling this integration is medical overmolding—a specialized form of multi-material injection molding used to combine soft and hard materials into a single, high-performance component. Whether for enhanced grip, integrated sealing, or aesthetic clarity, medical overmolding offers a reliable and validated route to durable, biocompatible solutions.
What Is Medical Overmolding?
Overmolding is the process of molding one material—typically a thermoplastic elastomer (TPE) or silicone—over another substrate, usually a rigid thermoplastic like polycarbonate (PC), polypropylene (PP), or ABS. In the medical field, overmolding is applied to create:
- Soft-touch grips on surgical or drug delivery devices
- Seals and gaskets integrated into housings or enclosures
- Color-coded or tactile identifiers for usability and compliance
- Vibration dampening or insulation features for diagnostic tools
- Transparent windows or optical components bonded to structural bodies
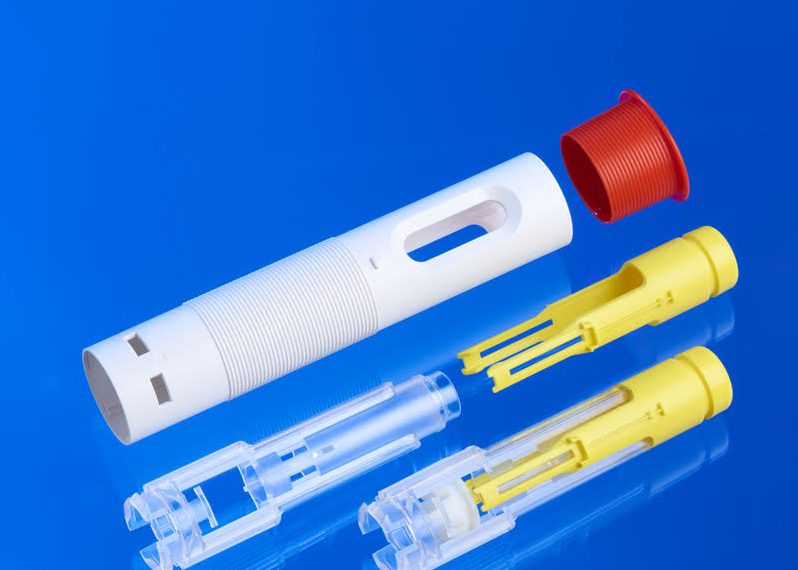
The process may be completed in a single machine (2K or multi-shot molding), or in separate steps, depending on design and volume.
Applications of Medical Overmolding
Overmolding is widely used across various sectors of medical technology:
| Sector | Example Applications |
|---|---|
| Drug Delivery | Auto-injectors, insulin pens, wearable pumps |
| Diagnostics | Microfluidic cartridges with integrated windows or seals |
| Surgical Instruments | Scalpels, trocars, laparoscopic handles |
| Ophthalmics | Dual-material contact lens packaging, IOL inserters |
| Wearables | Biosensor housings, patient interfaces, cable strain reliefs |
Materials Used in Medical Overmolding
Material compatibility is critical for overmolding success. Common pairings include:
- TPE over PC or ABS: For ergonomic grips or chemical-resistant surfaces
- Silicone over PEEK: For implantable or high-temperature applications
- COC over PP: For diagnostics with fluidic and optical functionality
- TPU over PC: For ruggedized and sterilizable enclosures
All materials must meet ISO 10993 or USP Class VI biocompatibility standards, and withstand sterilization methods such as gamma, EtO, or autoclave, depending on device classification.
Technical Considerations
Medical overmolding is more complex than standard injection molding. Key considerations include:
1. Adhesion Between Materials
Proper chemical and mechanical bonding is essential. Some polymers naturally adhere, while others require surface treatment (plasma, corona, or primers) to achieve durable interfacial bonding.
2. Tooling Design
Molds must be precisely engineered to support multi-shot processes or accommodate substrate loading without distortion. Flash control and venting are particularly critical in cleanroom environments.
3. Dimensional Control
Overmolding can introduce warpage or dimensional shift due to differing shrinkage rates. Moldflow simulations and precision tooling help compensate for these variables.
4. Cleanroom Integration
For Class I–III medical devices, overmolding often occurs in ISO Class 7 or 8 cleanrooms. Equipment must be validated, and all materials traceable per ISO 13485 standards.
Validation and Regulatory Compliance
As with any process in medical manufacturing, overmolding must be fully validated through:
- Design of Experiments (DOE)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Documented traceability and batch control
These are essential for compliance with ISO 13485, FDA QSR, and EU MDR requirements, especially for critical components.
Benefits of Medical Overmolding
- Enhanced Product Integrity – Reduces part count, risk of assembly failure, and contamination
- Improved Patient Experience – Ergonomic, soft-touch, and color-coded elements
- Sterilization Compatibility – Material bonding avoids adhesives that may degrade under sterilization
- Scalability – Automated molding is ideal for high-volume medical production
- Regulatory Efficiency – Fewer assemblies, less testing, and streamlined documentation
Overmolding Capabilities at Optimold
At Optimold, together with our sister company Micro Systems, we offer fully integrated medical overmolding services, from design through validation and production:
- Multi-shot and overmold tooling with sub-micron precision
- All-electric injection molding machines from 30t to 300t
- ISO Class 7 and 8 cleanroom molding
- Complete validation protocols (FAT, IQ, OQ, PQ)
- Global reach with UK and Singapore facilities
- ISO 13485 & ISO 9001 certified systems
Our capabilities support rapid prototyping and scalable production, offering OEMs a single partner for ultra-precision tooling and validated overmolding solutions.

Medical overmolding combines engineering precision, material science, and validated manufacturing to deliver high-performance components that are safer, stronger, and easier to use. As medical devices grow more sophisticated and patient-focused, overmolding stands out as a key technology that supports both innovation and compliance.
For OEMs looking to reduce risk, streamline design, and improve device functionality, overmolding is a smart and scalable solution—especially when supported by a trusted, ISO 13485-certified manufacturing partner.
Contact us today!